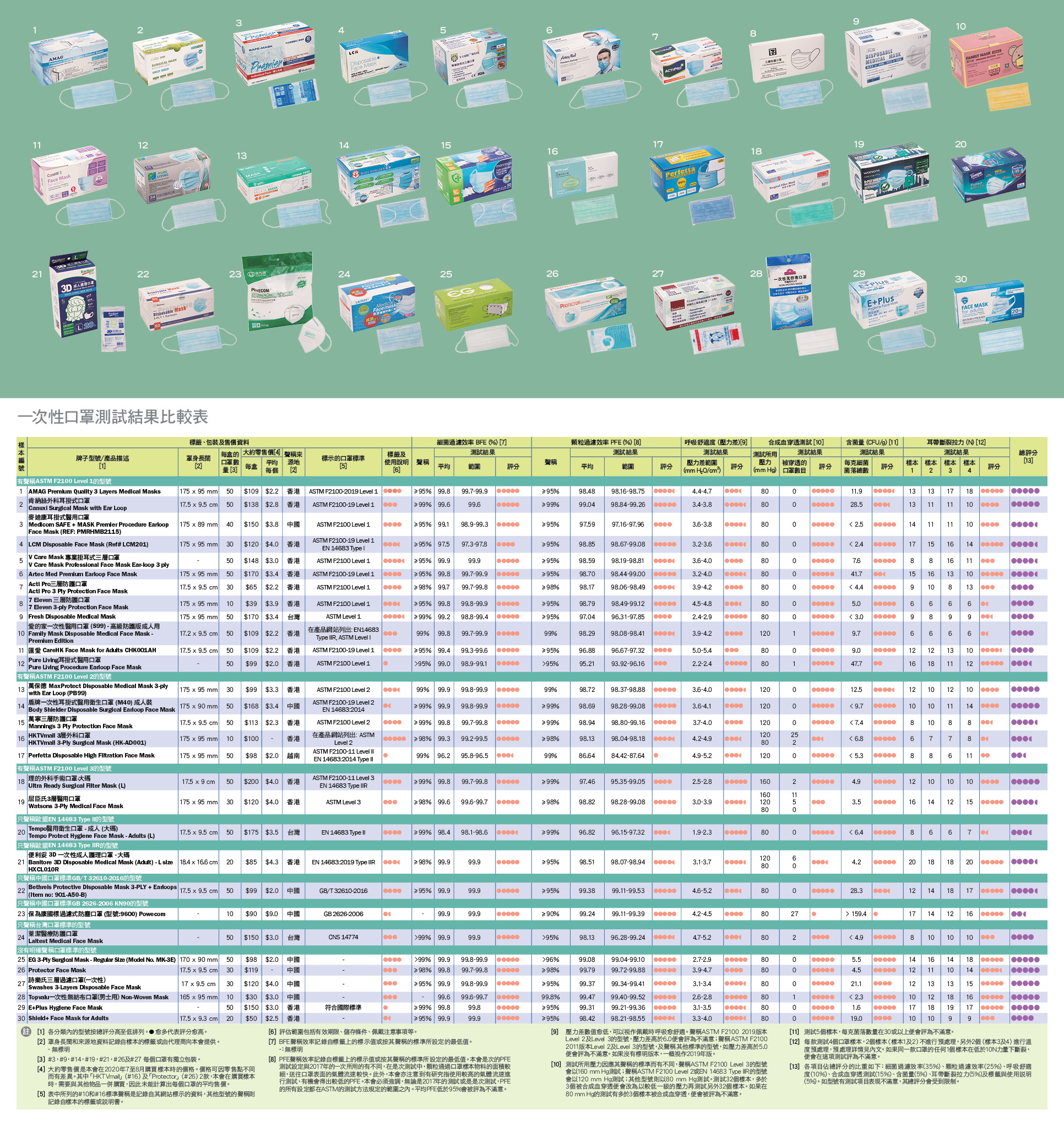
Test Samples
COVID-19 spread to Hong Kong in early 2020. The public started to panic-buy face masks. The Council paid close attention to the market situation and started planning on testing face masks again. Yet, due to shutdowns in some mainland cities, face masks were in short supply, and some retailers even had to limit purchase. Later, some local manufacturers started to produce face masks. With the resumption of work in various mainland cities, supply became stable in the middle of the year.
Between July and August 2020, as consumers, Council staff bought 30 face mask samples from medical supplies shops, dispensaries, houseware shops, convenience stores, supermarkets, department stores, cosmetics stores, and online shopping platforms.
When purchasing “HKTVmall” (#16) and “Protector” (#26), the Council had to buy other products such as hand rubs and wet tissues as a bundle and was hence unable to calculate the average retail price per face mask. Prices of other models ranged from $2.0 to $9.0 on average, with a 3.5 times difference between the lowest and highest prices. Yet, as the pandemic situation changes and so does the supply of face masks in the market, the prices of face masks may fluctuate continuously. Consumers should pay attention.
Regarding the place of origin, 18 models claimed to originate from Hong Kong, and others claimed to be from places including mainland China, Taiwan, and Vietnam.
Mask Standards on Sample Labelling
In the Council’s face mask report published in 2017, only about 20% of the samples indicated face mask standards. And among the samples in the current test, about 80% had indications of face mask standards. The most commonly used standard was Standard Specification for Performance of Materials Used in Medical Face Masks, ASTM F2100 by the American Society for Testing and Materials (ASTM). There were three different levels in this standard,.12 models claimed to have attained Level 1, 5 models claimed Level 2, and 2 models claimed Level 3. However, 2 models indicated ASTM Level 1 or ASTM Level 3 on their packaging labels, but without any indications of the standard number. The Council assumed that they claimed to be the ASTM F2100 specification. In addition, 2 models did not have indications of ASTM, and the claims on their websites only stated ASTM Level 1 or ASTM Level 2.
Similarly, the Council assumed they claimed to be the ASTM F2100 specification. Another standard used by more samples was the European Standard for Face Mask EN 14683, of which 7 models had indications. Among them, 5 models had indications of the American standard simultaneously. Moreover, 2 models mentioned the China National Standard and one the Taiwan Standard.
Test Items
After the Council’s face mask test report was published in 2017, ASTM Standard Specification for Performance of Materials Used in Medical Face Masks F2100 issued an updated version in 2019. The current test was conducted with reference to this new version. Apart from covering Bacterial Filtration Efficiency (BFE), Particle Filtration Efficiency (PFE), Differential Pressure and Test of Resistance to Penetration by Synthetic Blood already included in 2017, the EU EN 14683 Medical Face Masks - Requirements and Test Methods and China National Standard GB19083-2010 Technical Requirements for Protective Face Mask for Medical Use were also referred. Microbiological Content and Mask Harness Tension tests added. A laboratory in Hong Kong conducted the current test.
ASTM categorises face masks into three levels, and the test was based on its Level 1 requirements in assessing the usability of the samples for general community protection purposes. Among the tests mentioned above, Bacterial Filtration Efficiency and Particle Filtration Efficiency were the most important in determining the protective ability of face masks, and the Council hence assigned these two items higher weightings in calculating the overall ratings.
(Dr CHAN Tsz Tai, Co-chairperson of Advisory Committee on Communicable Diseases of the Hong Kong Medical Association, and Dr LIU Chung Ngar, Representative of the Hong Kong Medical Association and Specialist in Respiratory Medicine, contributed to some contents of this article.)
Test Methods and Results
Bacterial Filtration Efficiency (BFE)
BFE is an important indicator for assessing the protective efficacy of a surgical mask. Most standards for face masks have requirements in BFE. In ASTM F2100-19, the standard referred to in this round of mask test, the BFE must be at least 95% for Level 1 and at least 98% for both Level 2 and Level 3.
A liquid suspension of Staphylococcus aureus (S. aureus) was aerosolised with a mean particle size of around 3µm and delivered to a face mask sample using a constant flow rate and a given vapour pressure. The number of bacteria passing through the mask sample was collected with the agar plates using a 6-stage sampler. At the same time, the number of bacteria deposited was obtained under the "no mask" condition as a positive control. The BFE of each mask sample was obtained by comparing the number of bacteria passing through the samples and the positive control value. 5 samples of each model were tested.
Most tested models labelled their BFE claims with figures or indirectly made their BFE claims by stating the level of protection of the Standard they met. 10 models claimed to have a BFE of ≥95%, and 7 claimed to have a BFE of ≥99%. Moreover, quite a few models indicated in Chinese or English that the product "is highly effective in filtering bacteria" or "can serve as a barrier to bacteria and droplets" or similar descriptions.
The test results revealed that all models had an average BFE of over 95%. The overall performance was satisfactory. The best-performing ones were "V Care Mask" (#5), "Banitore" (#21), "Bethreis" (#22), "Powecom" (#23), "Laitest" (#24), and "Shield+" (#30). All 5 test samples of these 6 models reached a BFE of 99.9%.
3 models had at least 1 sample with a BFE lower than their claimed values, namely "Perfetta" (#17), "Tempo" (#20) and Fresh (#9). "Perfetta" (#17) claimed to have a BFE of 99%, and the measured BFE of its 5 samples ranged from 95.8% to 96.5%. "Tempo" (#20) claimed its BFE ot be 99%, but the measured BFE ranged from 98.1% to 98.6%, somewhat lower than its claim. Fresh (#9) claimed its BFE to be ≥99%, but one of its samples had a measured BFE of 98.8%, slightly lower than its claim.
BFE Claims
|
Level of BFE Claims |
Number of models |
| >95% | 1 |
| ≥95% | 10 |
| ≥98% | 5 |
| 99% | 3 |
| ≥99% | 7 |
| >99% | 2 |
| No indications | 2 |
Particle Filtration Efficiency (PFE)
PFE is another important indicator to assess the filtering efficiency of a face mask. PFE requirements of ASTM F2100-19 standard are at least 95% for Level 1 and at least 98% for both Level 2 and Level 3.
Polystyrene latex was aerosolised with a mean particle size of around 0.1µm, dried and delivered to a mask sample. A laser particle counter was used to count the number of particles penetrating the samples. For each model, 5 samples were tested. 2 samplings were conducted respectively before the particles passed through the sample's back (downstream) and front (upstream). Each sampling lasted 1 minute. The average number of particles penetrating the sample and the average number of particles delivered to where the mask was positioned as control were computed. The two values, namely the average number of particles passing through the mask and the control count were compared to calculate the PFE of each sample.
The test equipment settings in the current test were different from that conducted in 2017. In this test, the challenge particles were allowed to pass through a smaller area's face mask sample materials, and the airflow pass to the mask surface was also faster. In addition, the Council was aware of studies suggesting that a higher airflow rate used in tests might result in lower PFE levels. Therefore, even for samples of the same models and raw materials used, their PFE levels in the current and 2017 should not be directly compared. The Council did not compare the test results from the two tests. Consumers are advised to pay attention to this. The Council would like to stress that the PFE settings used in both tests were all within the ranges stipulated in the ASTM test method.
Most tested models labelled their PFE claims using figures or indirectly made their claims by stating the level of protection of the Standard they met. 15 models claimed to have a PFE of ≥95% or >95%, and 8 claimed to have a PFE of 99% or ≥99%. Moreover, 4 claimed to have a PFE of ≥98%. Another 3 models claimed to be >96%, 99.8% and ≥90%, respectively. Among the tested models, "Powecom" (#23) claimed to have a PFE of ≥90% and stated it was in compliance with the category KN90 of GB 2626-2006, a "respiratory protection—non-powered air-purifying particle respirator" standard of China. The PFE test method being adopted by the GB Standard was different from that of ASTM F2100.
The test results showed that the PFE levels of the majority were higher than 95%. The performance was satisfactory as a whole. The test model with the highest average PFE value was "Protector" (#26) with 99.79%, followed by "Topvlu" (#28) with 99.47%, "Bethreis" (#22) with 99.38% , "Swashes" (#27) with 99.37% , and "E+Plus" (#29) with 99.31%.

Yet, it was found that the measured PFE levels of at least 1 sample were lower than their claims in 10 models. Most of them claimed a relatively high PFE, such as 99%, ≥99%, or 99.8%. The model with the highest deviation between the test result and its claim was "Perfetta" (#17), the measured average PFE was 86.64% only, much lower than its labelled value 99%, as well as the requirement, stated in the ASTM Level 1 (PFE level of at least 95%). Its performance was disappointing.
PFE Claims
|
Level of PFE Claims |
Number of models |
| ≥90% | 1 |
| >95% | 2 |
| ≥95% | 13 |
| >96% | 1 |
| ≥98% | 4 |
| 99% | 3 |
| ≥99% | 5 |
| 99.8% | 1 |
Differential Pressure (ΔP)
A pressure meter was used to measure the pressure difference in the centre of the front and back sides of the mask sample under a constant flow rate. Differential pressure determines the breathability and comfortability of the wearers. The higher the measured value, the higher the breathing resistance. Referring to the requirements for Levels 2 and 3 of ASTM F2100-19 standard, the differential pressure must be under 6.0 mm H2O/cm2, and it should be under 5.0 mm H2O/cm2 for Level 1. These requirements were loosened than that in the 2011 version of ASTM F2100. Some models also described their comfortability when breathing using phrases such as "Comfortable and breathable", “Keep your breathing smooth”, and “Easy breathing”.
The test results showed that the differential pressure of all test samples of 6 models was all below 3.0 mm H2O/cm2, and they were “Fresh” (#9), “Pure Living” (#12), “Ultra Ready” (#18), “Tempo” (#20), “EG” (#25), and “Topvalu” (#28).
For another 4 models, some or all samples had a differential pressure higher than 5.0 mm H2O/cm2. They were “CareHK” (#11), “Perfetta” (#17), “Bethreis” (#22) and “Laitest” (#24). Their performance was relatively inferior. In particular, “Perfetta” (#17) labelled Level 2 of ASTM F2100 Standard (2011 version), while the differential pressure requirement for Level 2 was lower than 5.0 mm H2O/cm2, which was tighter than that of the 2019 version.
Resistance To Penetration By Synthetic Blood
The test on the resistance to penetration by synthetic blood simulates the splash of blood or body fluid during healthcare procedures by healthcare professionals to assess whether the surgical masks can provide sufficient protection for them in such incidence. In daily life, such incidence would be lower for the general public than that of healthcare professionals. Consumers may choose face masks that are fit for the anticipated risks. This test helps consumers understand the water resistance ability of each model, providing a certain reference value.
2ml of synthetic blood was spurted on each face mask sample from a distance of 30.5cm. An observation would be performed on whether the synthetic blood penetrated the mask sample to its backside within 10 seconds to assess its resistance to penetration by synthetic blood. The pressure applied in each test varied according to their claimed standard(s). Models claimed with ASTM F2100-19 Level 3 were tested using 160 mm Hg, while models declared with F2100-19 Level 2 or EN 14683 Type IIR were tested using 120 mm Hg, and the remaining tested with 80 mm Hg. 32 samples of each model were tested. If more than 3 samples were penetrated by synthetic blood, another 32 samples would be tested with a pressure one level lower. If more than 3 samples were found to be penetrated by synthetic blood in a test with 80 mm Hg pressure, the tested models would then be rated as unsatisfactory.
Test results suggested that no synthetic blood penetration was found in all 32 samples in 21 models. Their performance in this aspect was outstanding. 5 models had 1 or 2 samples penetrated by synthetic blood, which also met the requirement. 3 models were needed to conduct the test with a lower pressure, namely “HKTVmall” (#16), “Watsons” (#19), and “Banitore” (#21). In the higher pressure test, more than 3 of their 32 samples were penetrated by blood, resulting in a lower rating. In particular, “HKTVmall (#16) had 2 samples penetrated by synthetic blood in the 80 mm Hg test. The performance was unsatisfactory.
For this test item, the performance of “Powecom” (#23) was inferior. In the test using a pressure of 80 mm Hg, among the 32 samples, 27 of them were penetrated by synthetic blood. It may be due to its claim of an “anti-dust mask”. Regarding the claimed usage, it labelled that apart from “protection against dust and PM2.5 smog particulates” and also indicated wordings like “flu bacteria” and “droplets” on its package.
Bioburden
Wearing a face mask is to help to prevent infection, and therefore consumers should have a certain expectation on the hygiene of the face mask itself. Customs and Excise Department discovered in March and April 2020 that the bacterial counts of many face mask models were high and suggested that wearing face masks with an excessive amount of bacteria for a long time may lead to facial discomfort and may cause a potential health risk for people with impaired immune function. Therefore, the Council referred to the European Standard EN 14683 to test the bioburden of mask samples, extracting bacteria and fungi from them. 5 samples of each model were tested, and the averages were calculated.
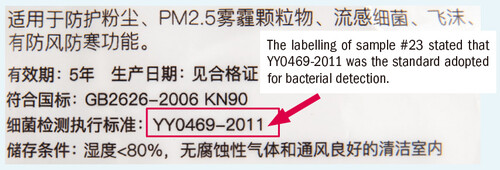
The coliform forming unit per gram in most tested models was lower than the limit set (CFU/g at less than 30) by the EU standard EN 14683. The overall performance was satisfactory. However, the CFU/g detected in 3 models were higher than 30, namely “Artec Med” (#6), “Pure Living” (#12), and “Powecom” (#23). Among them, the bacterial count detected in “Powecom” (#23) was >159.4CFU/g, it not only exceeded the limit of 30 under EN 14683 but also exceeded the hygienic requirements of the Mainland’s Industrial Standard for Surgical Mask YY0469-2011 and the limit for "Non-sterile mask" was 100 CFU/g.
Mask Harness Tension Test
The mask harness tension test was added to this test. Regarding the Mainland’s Standard GB19083-2010 “Technical Requirements for Protective Face Mask for Medical Use”, the test was to easily check whether the earloops would be detached from the main body of the face mask. For each model, 4 samples were tested, and 2 were pre-treated before being tested. First of all, they were placed in a test chamber under a high temperature of 70±3℃ for 24 hours, and then in a test chamber under a low temperature of -30±3℃ for 24 hours, and finally stored under room temperature for at least 4 hours. The remaining 2 samples need not be specially conditioned. A tension test device was used to measure the breaking strength of the junction of the straps and the main body of the face mask. If any 1 sample of the same model were torn apart at a tension of below 10N, they would be rated as unsatisfactory in this test.
The test results showed that 11 models had at least 1 sample torn apart at below 10N tension and were rated unsatisfactory. Among them, 5 models had the earloops of all 4 samples torn apart at below 10N, and they were “7 Eleven” (#8), “Fresh” (#9), “Family Mask” (#10), “HKTVmall” (#16), and “Tempo” (#20). A lower breaking strength means easier breakage. Consumers should carefully handle the earloops while putting on and taking off the face masks.
Price and Filtering Efficiency
The test results suggested that disposable face masks commonly available in the market were generally good quality. A BFE of 95% would be sufficient in providing reasonable protection. And the test results also revealed #1 and #25 were two models of excellent filtering efficiency and were comparatively affordable, averaging between $2.0 and $2.2 per piece (according to the prices of the samples in our sample purchases). The breathability of #25 was also outstanding. Apart from these, #28 would also be a very good choice with excellent filtering efficiency and breathability.
Some Models without Detailed User Instruction
In addition, the Council also examined the product packaging and discovered that the labelling information and user instructions of many models were insufficient. Information such as reminders to users regarding washing hands before putting on and after taking off the face mask, not touching the outward layer, replacing soiled and broken masks with a new one, and information about the proper way of discarding used masks were generally missed. For instance, the labelling information of “Perfetta” (#17) was written in Vietnamese, and no detailed information in Chinese or English; and “Pure Living” (#12), “Body Shielder” (#14), “Powecom” (#23), “E+Plus” (#29), and “Shield+” (#30) were other models without sufficient labelling information.
Wearing a Surgical Mask Properly
Before putting on a mask
- Wash your hands with soap and water or with an alcohol hand rub.
Steps to putting on a mask
- Refer to the product labels, and determine which side of the surgical mask faces outwards/inwards and upwards/downwards. The coloured-side faces out, and the metallic/plastic strip faces up. If the mask is not coloured, a downward folding pattern normally faces out.
- Extend the surgical mask so that the mask covers your mouth, nose, and chin completely.
- Position the earloops around both ears. For tie-on surgical masks, secure ties at the crown of the head and the nape.
- Mould the metallic strip over both sides of the nose bridge so that the mask fits snugly over the face.
- A common misuse is covering the mouth while exposing the nose.
While wearing a mask
- Try not to touch the mask with your hands as you might touch the bacteria or viruses. Users are advised not to take off their masks when speaking, coughing, or sneezing, which may spread the viruses.
- If the mask is wetted, for example, after coughing and sneezing, it should be taken off. After cleaning your hands, replace it with a new one. Do not re-use a used mask.
Taking off the mask
- When taking off the surgical mask, try not to touch the side facing out, as those areas might have been contaminated with bacteria or viruses.
- Do not randomly place the surgical mask or save it for re-use in your pocket.
- Throw the used surgical masks into a covered trash can as soon as possible and clean your hands immediately.
Other effective preventive measures
Apart from wearing a mask, we should adopt the following preventive measures:
- Hand hygiene is the most effective way to avoid the spread of diseases. We should always clean our hands properly, particularly before touching our eyes, nose and mouth. Wash your hands with soap and water when visibly soiled or contaminated with blood or body fluids. If the hands are not visibly soiled, one may clean them with 70-80% alcohol-based hand rub;
- Maintain cough manners and respiratory hygiene;
- Stay home and avoid contacting others if feeling unwell;
- Stay away from possible transmission sources: Reduce non-essential social activities and avoid visiting crowded places. If necessary, minimize the length of stay whenever possible. Moreover, persons susceptible to have infection-related complications, e.g., pregnant women or persons with chronic illnesses, are advised to wear surgical masks and avoid close contact with those infected as far as possible.
Surgical Mask
“Surgical masks” are generally treated as medical devices. Currently, there is no specific legislation in Hong Kong regulating the manufacturing, import, export, sale and use of medical devices. Yet, depending on the nature of the products and statements of related trade descriptions, these products may be regulated by existing legislation. Medical Device Division of the Department of Health is currently launching a voluntary “Medical Device Administrative Control System”, which includes General Medical Devices listed as Classes II, III, IV. As the surgical mask belongs to Class I General Medical Devices, it is therefore not under the “Medical Device Administrative Control System” listing.
The Council urges enhancing the regulation over surgical masks. This is not only due to the outbreak of COVID-19 diseases but also the health risk caused to the general public by the influenza epidemic surges every year. Properly wearing a surgical mask helps prevent infection. If the authorities enhance the regulation or establish clear guidelines, consumers can enjoy more peace of mind when purchasing and using surgical masks.
Recommendations by Customs and Excise Department
Customs and Excise Department (C&ED) launched a large-scale territory-wide special operation called “Guardian” on 27 January 2020, conducting inspections in various districts and examining surgical masks available for sale in the market to ensure that masks sold in the market complied with the Trade Descriptions Ordinance (TDO) and the Consumer Goods Safety Ordinance (CGSO). The main purpose of the inspection operation was to combat surgical masks alleging false origins, the inability to meet consumer goods safety standards, and forged trademarks. From 27 January 2020 to the publication of this magazine report, more than 6,200 Customs officers were mobilised to conduct over 39,000 inspections at various retail spots selling surgical masks, including chained stores, pharmacies, dispensers, and shops selling daily necessities.
Regarding the Council's test results, C&ED had already conducted follow up action with the provided information. Safety tests would be considered concerning the products' recognised standard(s) stated. Appropriate enforcement actions would be taken if any products were found to violate the TDO or the CGSO. According to the TDO, "trade descriptions", to goods, refer to an indication, direct or indirect, and in whatever form and by whatever means, for goods or any parts of the goods, including claims regarding fitness for purpose, strength, performance, and compliance to any standards. The TDO does not make it mandatory to specify the information on goods or their packaging. Yet, in trade or business, all trade descriptions marked on or attached to goods must be true and correct, not misleading. Traders' making a false or misleading statement about goods without solid supporting evidence may constitute an offence of false trade description. Violation of the TDO is a serious offence. Upon conviction, the maximum penalty is a fine of $500,000 and imprisonment for five years. In addition, the safety of consumer goods that are ordinarily supplied for private use in Hong Kong, if not covered by other legislation, is subject to the regulation of the CGSO. According to the CGSO, manufacturers, importers and suppliers must ensure that the consumer goods comply with the "general safety requirement", which is reasonably safe. It is an offence to import, manufacture or supply consumer goods unable to comply with the general safety requirements. The maximum penalty upon conviction is a fine of $100,000 and imprisonment for one year on the first conviction, and $500,000 and imprisonment for two years on subsequent convictions.
Wearing a Face Mask Properly Helps to Prevent Infection
The Department of Health suggested that COVID-19 and common upper respiratory tract infections are mostly transmitted through droplets. Also, most of the surgical masks adopted a three-layer design which includes an outer fluid-repelling layer, a middle layer that serves as a barrier to germs, and an inner moisture-/water-absorbing layer. Wearing a mask can thus protect ourselves and prevent the spread of infection to others. Numerous scientific studies suggested that wearing a mask can prevent infection. Medical professionals from World Health Organization, the US and European countries all supported such recommendations is conducive to fighting the epidemic.
The Hong Kong Medical Association pointed out that according to a literature review published in the June 2020 issue of the medical journal, "The Lancet", masks can greatly reduce the risk of infection. Another journal, "Nature (Medicine)", also conducted tests on surgical masks in 2020, and their results indicated that masks could reduce respiratory droplets and virus nucleic acid detected in aerosols. When purchasing a mask, users should pay attention to the filtering efficiency of the materials, whether they might cause allergies, the expiry date and their storage condition. It is also very important to check whether the shape and size of the mask are suitable for the user. They should also pay attention to the presence of a metallic strip to help fit the mask snugly over the face. However, very tight-fitting masks (such as N-95) might not be the best for the general public, as misuse might cause face skin irritation and discomfort and turn off the users from wearing them.
BFE and PFE
Manufacturer of “Canuxi” (#2) submitted to the Council a test report on their products manufactured after 1 June 2020 and claimed that their sample(s) reached a PFE of ≥99%.
Agent of “Fresh” (#9) suggested that although the Council’s test discovered the BFE of 1 sample was lower than their claims of 99%, they opined that it might be due to the measurement deviation. The company submitted a relevant test report and claimed that the test results obtained by the manufacturer were all ≥99%.
Agent of “Body Shielder” (#14) submitted a test report to the Council and claimed that the PFE of their product was 99.93%.
Agent of “Ultra Ready” (#18) suggested that the Council’s test result on the PFE test was different from their own tests commissioned to a laboratory in the US since 2000. After looking into the matter, it was discovered that the Council commissioned a Hong Kong laboratory for this test, while the laboratory commissioned by the Council in 2017 was the same as the one used by the company. They stated that the Hong Kong laboratory started providing test service on PFE tests since July 2020, and the test conditions adopted (such as test area, face velocity, and airflow rate) were different from that being used by the laboratory in the US, which possessed over 20 years of experience. They conducted their internal research and compared the test results obtained from different laboratories, and they suggested that the test methods would influence the test results. For example, in comparing the test methods and results of both laboratories, they suggested that the PFE test methods used by the Hong Kong laboratory would cause a reduction in PFE with a reduction ranging from 0.77% and 1.9%. They also stated that the thinner the material used, the higher the reduction level. At the same time, they employed another laboratory in Hong Kong to conduct a related study, which also supported their argument. Based on their studies abovementioned, the supplier suggested that the methods used in the Council’s test conducted in Hong Kong would cause a reduction in the measured PFE values. As a result, The Council’s magazine report might be misleading to the consumers, and the comparison might not be as fair and reasonable as the previous tests. (Council’s remarks: The company submitted 3 sets of test reports, which compared the Council’s test results and the ones obtained by their US laboratory. In contrast, the 3 sets of test data obtained by the Hong Kong laboratory were lower than that of the US laboratory by 0.77%, 0.13% and 0.33%, respectively. The PFE settings used in the Council’s laboratory were well within the acceptable ranges allowed by the ASTM standard, and the test methods adopted were also accredited.)
Agent of “Tempo” (#20) submitted to the Council a relevant test report of comprehensive testing on the product, with all results indicating that its BFE and PFE were both above 99%. From the company’s understanding, test results about the filtering efficiency might be influenced by many factors, including but not limited to temperature and humidity of the environment, pulling by external forces, UV exposure, etc. They explained that the
mask samples might have been affected by the above factors during transportation or storage, resulting in deviations in individual test samples. They expressed a dedication to maintaining product quality, ensuring that the public could feel at ease when using.
Breathability
Agent of “Laitest” (#24) submitted to the Council a relevant test report by the manufacturer, which included multiple test parameters. Among them, their differential pressure test results were all within the range of 2.8 mm H2O/cm2 to 3.2 mm H2O/cm2.
Resistance to Penetration by Synthetic Blood
The manufacturer of “HKTVmall” (#16) suggested that their mask products were not designed for medical use but rather in the hope to provide reliable protection for the general public. Since the resistance to penetration by synthetic blood test conducted by the Council differed from their test conducted in April 2020, they revealed that they had conducted another test in November. 140 mask samples were randomly selected and tested, and test results showed that they met the standard. They claimed that the chosen samples were made of the materials and the equipment same as that of the samples batch tested by the Council.
The manufacturer of “Watsons” (#19) did not agree to the test results of the Council. After receiving the Council’s test report, they arranged their own test. They hired the same laboratory as the Council to perform another resistance to penetration test on the same batch of products. Their test results suggested that their mask samples met the ASTM Level 3 standards, which matched their previous test results, including reports from April, May and June 2020. They opined that there were sufficient reasons to be sceptical about the Council’s test results. They opined that the laboratory commissioned by the Council had their testing set up in Hong Kong for less than 6 months. The same laboratory was employed for similar tests with the same parameters and methods for the company, they had tested twice for the company with mask products made of the same raw materials and produced under the same production environment, and test results revealed that their samples passed the resistance to penetration by a synthetic blood test, and achieved ASTM Level 3standard. In addition, they also indicated that the laboratory was the only laboratory commissioned by the Council for the current face mask test, and the report was only based on one test conducted on that particular batch of face mask samples. They were, therefore, sceptical about the conclusions drawn from the test. They also indicated that it had commissioned three independent professional test organisations in Hong Kong to conduct examinations for them. All reports proved that the batch of masks reached ASTM Level 3 standard. (The Council’s remarks: The laboratory performing the test for the Council used methods following the ASTM standard, and the methods had also been accredited.) Manufacturer of “Watsons” emphasised that ever since it started producing face masks, they had always followed a stringent quality inspection process of high specifications. Raw material supply, clean room production line, and retail spots had to undergo quality inspection of 9 items regularly. Apart from maintaining stringent quality
control measures, they also regularly invited different independent laboratories to conduct tests on their mask samples. Through multiple and intensive tests, instead of relying on a single test result by one laboratory, they claimed that their masks met the quality standards and that users could feel at ease using them. As for suggestions regarding labelling information and user instructions, they would further examine and follow up.
Agent of “Banitore” (#21) submitted a test report to the Council and suggested that their products underwent two resistance to penetration by synthetic blood tests. Results of both met the standard requirements. The company would purchase samples from the market of similar manufacturing dates to perform another test. As for the problem of labelling information, they indicated that instructions would be added.
Earloops
Agents of “Acti Pro” (#7), “7-Eleven” (#8) and “Mannings” (#15) revealed that their products had always strictly followed the ASTM standard. They also submitted test reports issued by independent laboratories they commissioned to test the breaking strength of the junction of their face masks, and their results showed that the relevant standards were met.
Agent of “Fresh” (#9) claimed that tests on breaking strength of the junction of face masks in Taiwan adopted a self-management approach, and earloop was tested by hand instead, following a principle that it would not be torn apart when extended for about 1.5 times its length. Also, considering the comfortability of wearing, earloops were attached to the inside, and the elastic earloops were further supported by a piece of 20mm side cloth to enhance their fastness.
Agent of “Family Mask” (#10) stated that the mask samples tested by the Council were produced in June 2020. They claimed that the earloops were made of materials of 45 grams per square metres in earlier days of production, and the design should meet the tension requirement theoretically. As it was discovered that the material stability needed to be improved, earloops had been made of materials of 90 grams per square metres since August 2020, which in turn doubled the tension. They opined that there would not be any problems even when the breaking tension was under 10N under normal wearing. In addition, they submitted a test report to the Council and claimed that their product met ASTM Level 1 and EN 14683 Type IIR requirements, and the PFE was also higher than 99%. Hence they made a related claim.
Agent of “Shield+” (#30)alleged that their product was produced according to ISO 13485 Medical Devices Quality Management System standards. Referring to the tests conducted by their manufacturer, the test was conducted under room temperature environment (21℃+/-5℃), and the breaking tension was no less than 10N. Product meeting this standard should be suitable for Hong Kong consumers under normal circumstances. However, the tension test conducted by the Council was referred to GB 19083 standard, in which two samples were not specially pre-conditioned, the test result was 10N, rated as “pass”. They had reservations about the test results of 9N for the other two pre-conditioned samples rated as “fail”. They opined that the pre-conditioning environmental temperatures of 70℃ and -30℃ were not ideal ranges and conditions for consumers to use or store masks. Their masks were not designed for use or stored under extreme environmental temperatures.
Bioburden
Manufacturer of “Artec Med” (#6) submitted to the Council a relevant test report, stating that the bioburden of its product complied with the standard. They questioned the recovery efficiency in the Council’s test was too low, and large differences in bacterial counts among different samples were recorded. They opined that the samples might have been contaminated beforehand, and the test results were unfair, unsafe, inconclusive, and inaccurate.
Manufacturer of “Pure Living” (#12) submitted relevant certificates and test reports to the Council and stated that their products had been awarded the Q-Mark Product Certification. Moreover, they also claimed that the face masks they produced had been awarded certification from the EU, proving that the product quality had attained passing levels. Regarding the problems of excessive bacterial count and unstable test data, they believed that many factors such as the storage environment, temperature, buying channels of the test samples, and test procedures should have certain impacts. The company also claimed that improvements had been made regarding the batch number and expiry date on the product label.
Labelling and Instructions for Use
The manufacturer of “Canuxi” (#2) submitted the design sketch of its new box packaging and stated that the information on labelling and instructions for use would be revised.
Agent of “Medicom” (#3) indicated that they would regularly share instructions for using protective products and user manuals of infection prevention, including steps to properly use a mask on their website or social media platforms. They would also consider printing such information on product packaging in the future.
Agents of “Family Mask” (#10) and “EG” (#25) both indicated that they would enhance the content of their instructions for use.
The manufacturer of “E+Plus” (#29) sent their new box packaging and indicated that the packaging had already been changed.
Source of samples
Supplier of “Perfetta” (#17) submitted a manufacturer certificate to the Council stating that their two products reached ASTM Level 3 and EN 14683 Type IIR levels. And another supplier of “Perfetta” suggested that the test samples tested by the Council were not products of their company supplied in Hong Kong and suspected that the Council’s samples were from parallel imports and defective, or products infringing Perfetta’s trademark. The company opined that the Council should not publish test results using the said samples not to create confusion and panic.
Agent of “Powecom” (#23) stated that the samples tested by the Council were “Powecom” 9600 Particulate Respirator, which was packaged in the Mainland and the National Standard GB2626-2006 was applied. The product was supposed to be for domestic sales in the Mainland and, therefore, not in the Hong Kong market packaging, which should bear Chinese and English labelling. They opined that perhaps due to the pandemic this year, unknown companies had imported their products from the Mainland for sale in Hong Kong, and they were not sold directly by them (the agent).
Price
The manufacturer of “Canuxi” (#2) stated that its retail price on 16 November 2020 was $60.
Agent of “Acti Pro” (#7) suggested that the retail price of each pack (30 pieces) during the second week of November 2020 was $60.
Agent of “7-Eleven” (#8) suggested that the retail price of each pack (10 pieces) during the second week of November 2020 was $29.
Agent of “Body Shielder” (#14) stated that the latest retail price on 14 November 2020 was $80 for 50 pieces (individual package).
Agent of “Mannings” (#15) suggested that the retail price of each pack (50 pieces) during the second week of November 2020 was $78.
The manufacturer of “HKTVmall” ($16) suggested that the retail price was $25 for 10 pieces.
Agent of “Bethreis” (#22) stated that the retail price of each box was $49.







![[Baby Snacks Guide] Who Says Snacks Can’t Be Healthy?](/f/guide_detail/415742/376c212/bb%20snack.jpg)


