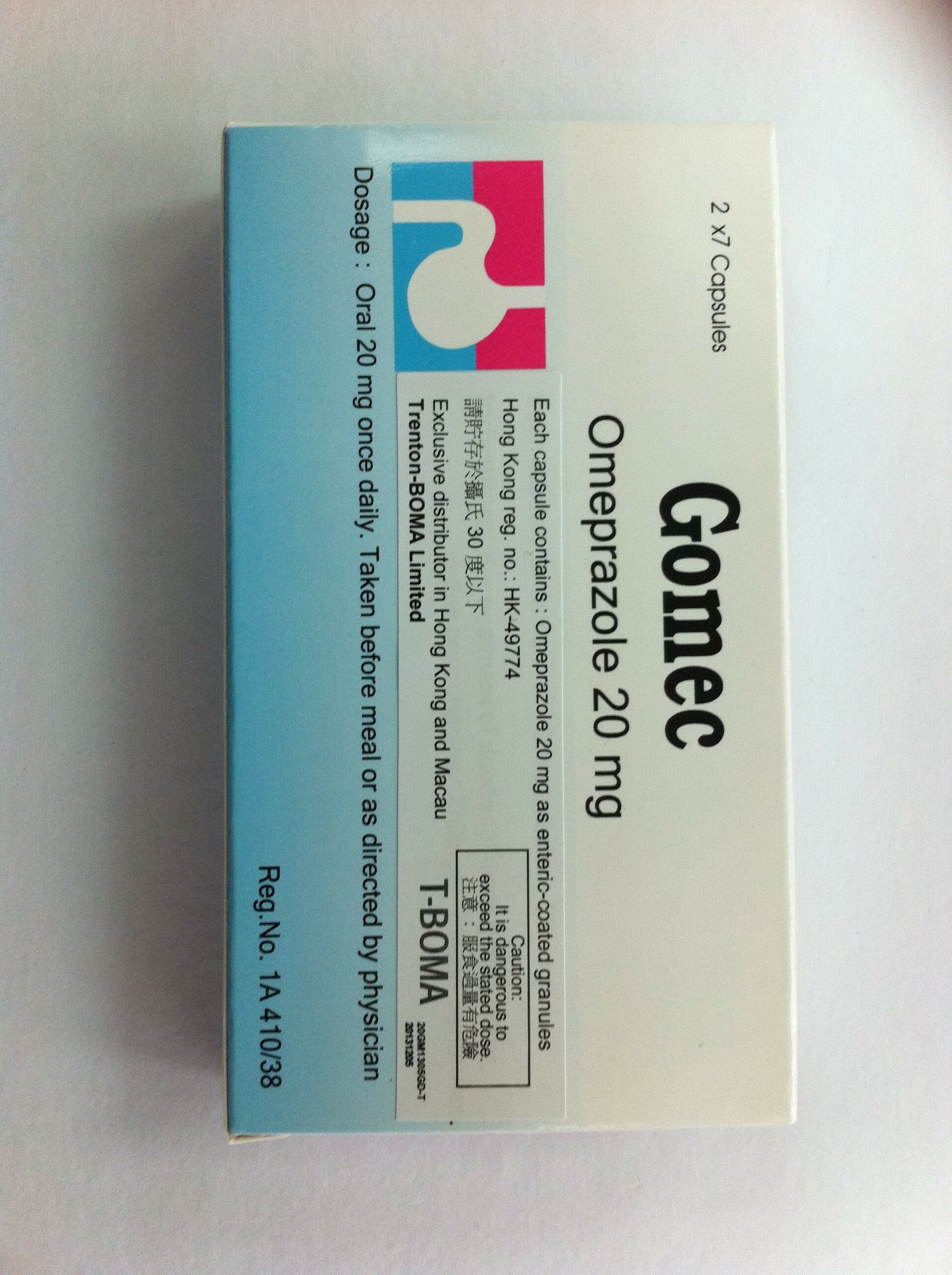
The Department of Health (DH) today (February 10) instructed a licensed drug wholesaler, Trenton-Boma Company Limited (Trenton-Boma), to recall one batch of Gomec 20mg Capsules (Gomec 20mg) (registration number: HK-49774, batch number: 10230) from shelves. The recall was due to some samples of the above batch drawn from the market by DH were found to have failed the dissolution test. So far, there is no evidence suggesting that other batches are affected. The affected batch will expire by June 2014.
"Gomec 20mg, containing omeprazole, is a Part I poison used for the treatment of peptic ulcer and gastric hyperactivity. It can only be supplied by pharmacies under the supervision of a registered pharmacist," a spokesman for the DH explained.
Trenton-Boma has set up a hotline (8101 2716) for related enquiries. DH will closely monitor the recall.
"Members of the public who are using the affected batch of the product should consult their healthcare professionals if in doubt," the spokesman advised.
- 2014.02.10