The Department of Health (DH) today (October 10) endorsed a licensed drug wholesaler, Julius Chen & Company (HK) Limited (Julius Chen), to recall a batch (batch number: 1012220026) of PROSOME Enteric Coated Tablets 20mg (Hong Kong Registration Number: HK-58864) from the market due to a quality issue.
In the course of a routine market surveillance by the DH, samples of the product from the above batch were collected for analysis. Test results from the Government Laboratory showed that the samples had failed the disintegration test. Julius Chen was informed of the test result and thus recalled the relevant batch of product from the market. The DH's investigation is continuing.
The above product, containing esomeprazole, is a prescription-only medicine indicated for peptic ulcer disease and gastro-oesophageal reflux disease. According to Julius Chen, the product has been supplied to local pharmacies and private doctors.
Julius Chen has set up a hotline (2487 1301) to answer related enquiries. The DH will closely monitor the recall.
So far, the DH has not received any adverse reaction reports in connection with the above product.
Patients who are taking the above product should not stop taking the medicine, but should consult their healthcare professionals as soon as possible for appropriate arrangements.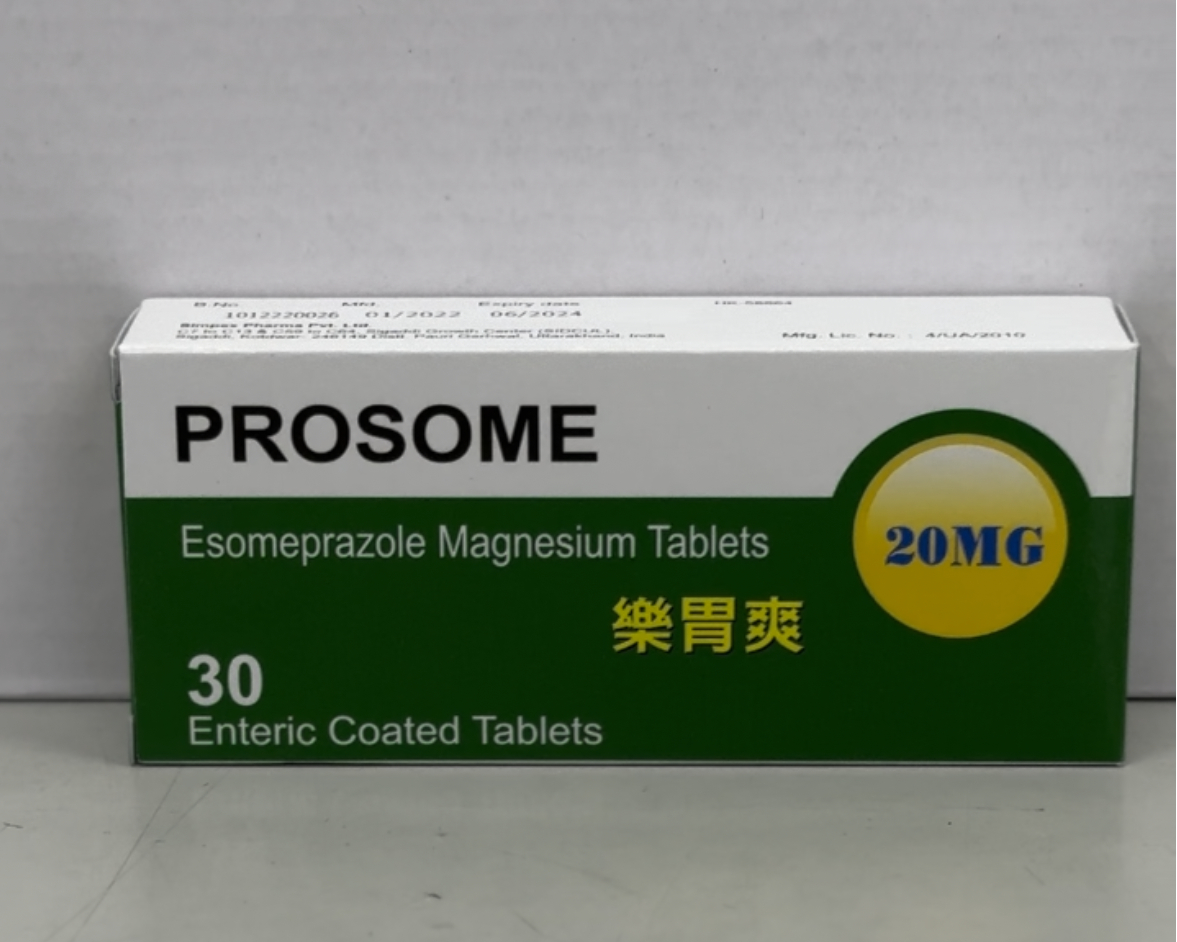
Reposted from HKSAR Government webpage:
https://www.info.gov.hk/gia/general/202310/10/P2023101000540.htm
- 2023.10.10


