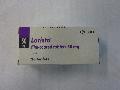
 The Department of Health (DH) today (April 30) instructed Unipharm Trading Co. (Unipharm), a licensed wholesaler, to recall from market all batches of Lorista tablets 50mg (HK-59349) as the product may bear unapproved package insert and render it unregistered pharmaceutical product.
The Department of Health (DH) today (April 30) instructed Unipharm Trading Co. (Unipharm), a licensed wholesaler, to recall from market all batches of Lorista tablets 50mg (HK-59349) as the product may bear unapproved package insert and render it unregistered pharmaceutical product.
A DH spokesman revealed that the matter came to light upon the department's investigation into a public enquiry that the package insert was in a language not correspond to the registered version.
According to the DH investigation so far, all the products at the warehouse of Unipharm were found to contain unregistered version of package insert. Therefore, Unipharm was instructed to recall all the products from the market.
DH investigation continues.
Unipharm had so far imported 5,500 boxes, among which, 2,209 boxes had been sold to private doctors and local pharmacies.
Lorista contains losartan which is for the treatment of hypertension. It can only be sold on prescription and under the supervision of pharmacists in registered pharmacies.
"Here, contravention of the Pharmacy and Poisons Ordinance is suspected. The sale and possession of unregistered pharmaceutical products is an offence under the Pharmacy and Poisons Ordinance. The maximum penalty is a fine of $100,000 and two years' imprisonment," the spokesman explains.
Unipharm has set up a hotline (2499 1373) for the recall. DH will monitor the recall.
(Reprinted from HKSAR Government web page
http://www.info.gov.hk/gia/general/201204/30/P201204300572.htm )


