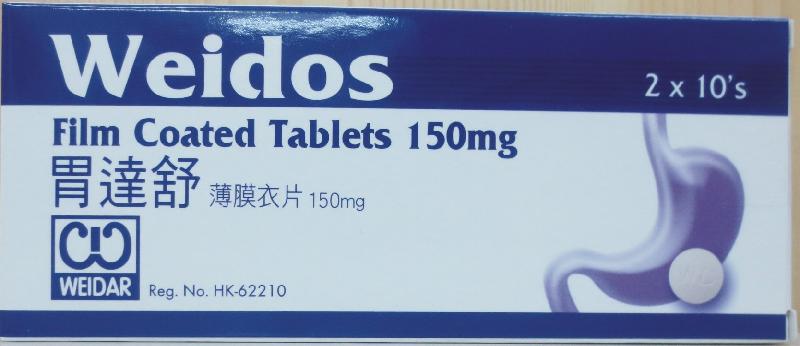
The Department of Health (DH) today (September 30) endorsed a licensed drug wholesaler, Vast Resources Pharmaceutical Limited, to recall a ranitidine-containing product, namely Weidos Tablets 150mg (Hong Kong registration number: HK-62210), from the market due to the presence of an impurity in the product.
The DH received notification from Vast Resources today that the product's active ingredient was found to contain low levels of N-nitrosodimethylamine (NDMA). NDMA is classified as a probable human carcinogen based on results from laboratory tests. As a precautionary measure, Vast Resources voluntarily recalled the affected product from the market.
The DH, via its surveillance system, was aware that certain ranitidine-containing products were found to contain NDMA in other countries and had been collecting samples of ranitidine-containing products from the market for analysis. The DH also noted that overseas drug regulatory authorities including the United States Food and Drug Administration and the European Medicines Agency have been reviewing the safety impact of the impurity found in the ranitidine-containing products. The DH will closely monitor the development of the issue and any safety update of the drug issued by overseas drug regulatory authorities for consideration of any action deemed necessary. A letter was also sent to healthcare professionals on September 18 notifying them about the issue.
The above product is an over-the-counter medicine used for the treatment of gastric diseases. According to Vast Resources, the product has been supplied to local private doctors, pharmacies and medicine stores, and some has been exported to Macao.
Vast Resources has set up a hotline (2539 0990) to answer related enquiries.
"So far, the DH has not received any adverse reaction report in connection with the product. The DH will closely monitor the recall," a spokesman for the DH said.
"Patients who are taking the above product should seek advice from their healthcare professionals for appropriate arrangements. There are alternative medicines available on the market with similar indications," the spokesman added.
Reposted from HKSAR Government webpage:
https://www.info.gov.hk/gia/general/201909/30/P2019093000647.htm