The Department of Health (DH) today (May 13) endorsed a licensed drug wholesaler, Hind Wing Co Ltd (Hind Wing), to recall one batch (batch number: 1709191/2) of Tepadina Powder for Infusion 100mg (Hong Kong Registration Number: HK-63411) from the market as a precautionary measure due to a potential defect of the product.
The DH received notification from Hind Wing today that the vial seal and closure may be defective in the affected batch. This might affect the quality of the product. As a precautionary measure, Hind Wing is voluntarily recalling the affected batch from the market.
The product, containing thiotepa, is a prescription medicine used for the treatment of various cancers. According to Hind Wing, the affected batch has been supplied to the Hospital Authority, a private hospital and pharmacies.
Hind Wing has set up a hotline (2541 5731) to answer related enquiries.
"So far, the DH has not received any adverse reaction reports in connection with the product. The DH will closely monitor the recall," a spokesman for the DH said.
"Members of the public should consult healthcare professionals if in doubt or feeling unwell after using the product," the spokesman added.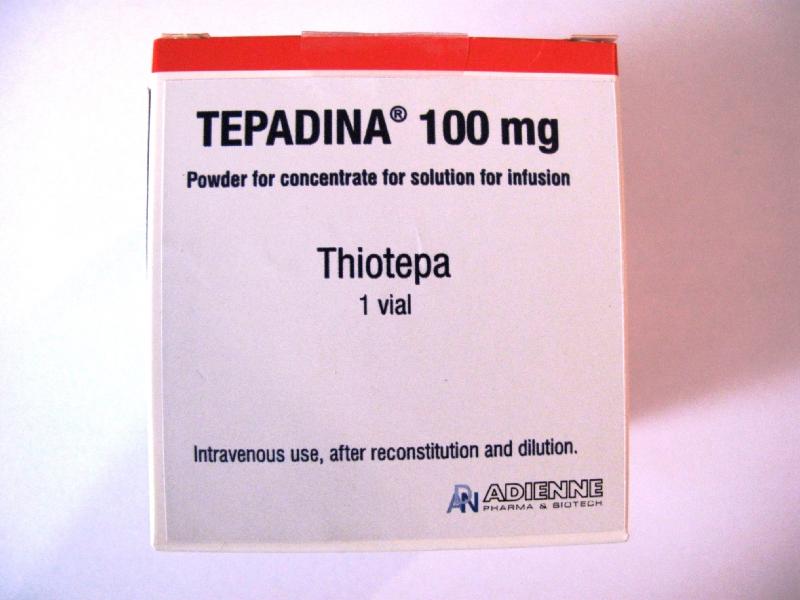
Reposted from HKSAR Government webpage:
https://www.info.gov.hk/gia/general/202005/13/P2020051300605.htm
- 2020.05.13



